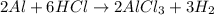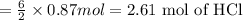Chemistry, 18.05.2021 19:20 raymondleggett44
Aluminum reacts with hydrochloric acid to produce aluminum chloride and hydrogen. Write a balanced equation for the reaction and calculate the number of moles of hydrochloric acid required to react with 0.87 mole of aluminum.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 22:00
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2

Chemistry, 23.06.2019 00:30
There are approximately 15 milliliters (ml) in 1 tablespoon (tbsp). what expression can be used to find the approximate number of milliliters in 3 tbsp?
Answers: 1
You know the right answer?
Aluminum reacts with hydrochloric acid to produce aluminum chloride and hydrogen. Write a balanced e...
Questions

Mathematics, 06.11.2019 08:31

Biology, 06.11.2019 08:31

Spanish, 06.11.2019 08:31

Mathematics, 06.11.2019 08:31




Arts, 06.11.2019 08:31

Biology, 06.11.2019 08:31

Business, 06.11.2019 08:31

History, 06.11.2019 08:31

Mathematics, 06.11.2019 08:31

Mathematics, 06.11.2019 08:31

Mathematics, 06.11.2019 08:31

Mathematics, 06.11.2019 08:31

Mathematics, 06.11.2019 08:31

Mathematics, 06.11.2019 08:31

History, 06.11.2019 08:31


History, 06.11.2019 08:31