

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:40
Achemistry student weighs out of phosphoric acid , a triprotic acid, into a volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with solution. calculate the volume of solution the student will need to add to reach the final equivalence point. round your answer to significant digits.
Answers: 3

Chemistry, 22.06.2019 01:00
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1

You know the right answer?
Write the net ionic equation for the reaction that occurs when equal volumes of 0.062 M aqueous form...
Questions


English, 03.05.2021 20:20


SAT, 03.05.2021 20:20

English, 03.05.2021 20:20


Spanish, 03.05.2021 20:20



History, 03.05.2021 20:20


Mathematics, 03.05.2021 20:20

Social Studies, 03.05.2021 20:20





Mathematics, 03.05.2021 20:20

Mathematics, 03.05.2021 20:20

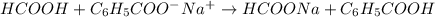
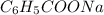 .
. 

