Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 06:40
Three alkali metals in group 1 are a. calcium, strontium, barium b. boron, aluminum, gallium c. sodium, potassium, rubidium d. fluorine, iodine, chlorine
Answers: 1

Chemistry, 22.06.2019 12:00
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3
You know the right answer?
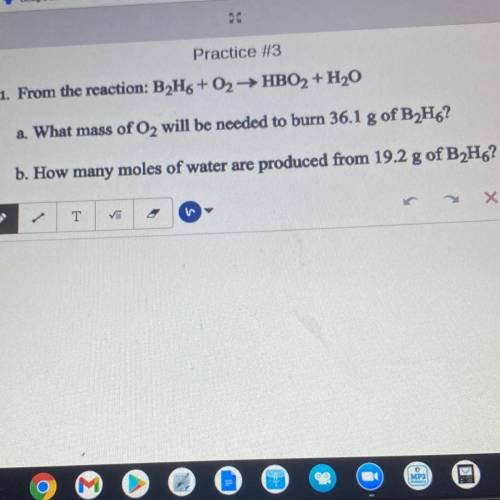
. From the reaction: B2H6+02HBO2 + H20
2. What mass of O2 will be needed to burn 36.1 g of B2H6?
Questions





Mathematics, 11.10.2019 19:00


Mathematics, 11.10.2019 19:00


Mathematics, 11.10.2019 19:00

Social Studies, 11.10.2019 19:00

Spanish, 11.10.2019 19:00


History, 11.10.2019 19:00

Social Studies, 11.10.2019 19:00



Mathematics, 11.10.2019 19:00


Business, 11.10.2019 19:00

Spanish, 11.10.2019 19:00