
Chemistry, 19.05.2021 01:00 estefaniapenalo
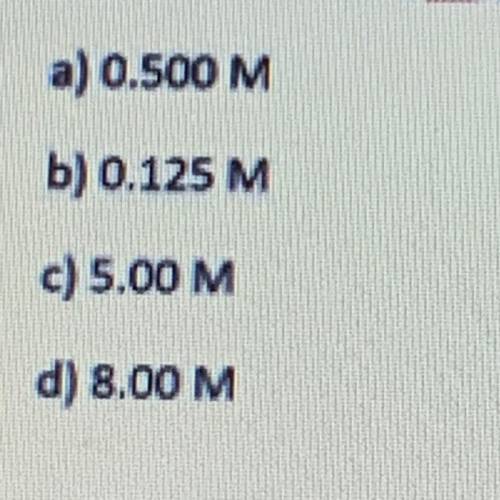
A volume of 20.0 of 0.250 M neutralizes a 40.0 sample of KOH. What is the concentration of кон? HCl(aq)+KOH(aq) KCl(aq)+H 2 O(l)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3


Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1
You know the right answer?
A volume of 20.0 of 0.250 M neutralizes a 40.0 sample of KOH. What is the concentration of кон? HCl(...
Questions


Biology, 18.10.2021 07:10

Mathematics, 18.10.2021 07:10





Mathematics, 18.10.2021 07:10




Mathematics, 18.10.2021 07:10




Arts, 18.10.2021 07:10






