2
3 K 4
KM
5
67
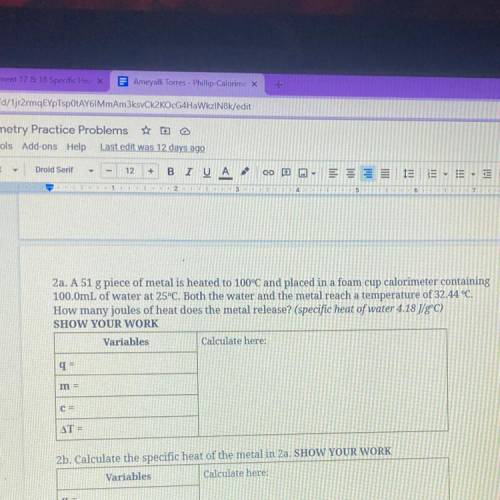
2a. A 51 g piece of metal is heated to 100°C and placed in...

Chemistry, 19.05.2021 01:00 morenoozzie
2
3 K 4
KM
5
67
2a. A 51 g piece of metal is heated to 100°C and placed in a foam cup calorimeter containing
100.0mL of water at 25°C. Both the water and the metal reach a temperature of 32.44 °C.
How many joules of heat does the metal release? (specific heat of water 4.18 J/gºC)
SHOW YOUR WORK
Variables
Calculate here:
q=
m =
C=
AT =

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:00
What is important to study for nios grade 12 chemistry? i have only one month left.
Answers: 2

Chemistry, 21.06.2019 22:30
Omg imgonnafailnfiedkla use complete sentences to explain how the mass of hydrogen is conserved during cellular respiration.
Answers: 1

Chemistry, 22.06.2019 00:10
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 2
You know the right answer?
Questions

Mathematics, 05.02.2021 04:00

Mathematics, 05.02.2021 04:00

English, 05.02.2021 04:00


Mathematics, 05.02.2021 04:00

Mathematics, 05.02.2021 04:00


Advanced Placement (AP), 05.02.2021 04:00

English, 05.02.2021 04:00

Mathematics, 05.02.2021 04:00

English, 05.02.2021 04:00

English, 05.02.2021 04:00


Chemistry, 05.02.2021 04:00

Mathematics, 05.02.2021 04:00


Mathematics, 05.02.2021 04:00

Computers and Technology, 05.02.2021 04:00

Mathematics, 05.02.2021 04:00



