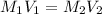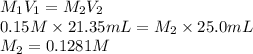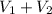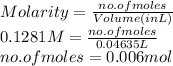Chemistry, 19.05.2021 14:00 jazminpratt0311
In an acid-base titration, a student uses 21.35 mL of 0.150 M NaOH to neutralize 25.00 mL of H2SO4. How many moles of acid are in the flask?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1

Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1

Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1
You know the right answer?
In an acid-base titration, a student uses 21.35 mL of 0.150 M NaOH to neutralize 25.00 mL of H2SO4....
Questions


Mathematics, 11.10.2019 05:30



Physics, 11.10.2019 05:30



History, 11.10.2019 05:30

History, 11.10.2019 05:30






Mathematics, 11.10.2019 05:30

Mathematics, 11.10.2019 05:30

Mathematics, 11.10.2019 05:30

Mathematics, 11.10.2019 05:30

Spanish, 11.10.2019 05:30

SAT, 11.10.2019 05:30

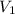 = 21.35 mL,
= 21.35 mL, 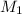 = 0.150 M
= 0.150 M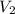 = 25.0 mL,
= 25.0 mL, 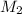 = ?
= ?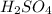 is as follows.
is as follows.