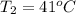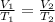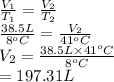An arctic weather balloon is filled with 38.5 L of helium gas inside a prep shed. The temperature inside the shed is 8. °C. The balloon is then taken outside, where the temperature is -41. °C. Calculate the new volume of the balloon. You may assume the pressure on the balloon stays constant at exactly 1 atm. Be sure your answer has the correct number of significant digits.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 12:30
Acycloalkane molecule contains 8 carbon atoms. how many hydrogen atoms are present in the molecule?
Answers: 2


Chemistry, 22.06.2019 09:00
Chemical energy is a form of a. kinetic energy only. b. both potential and kinetic energy. c. neither potential nor kinetic energy. d. potential energy only. reset
Answers: 1

Chemistry, 23.06.2019 03:30
Name atleast 3 type of energy associated with the microwave
Answers: 1
You know the right answer?
An arctic weather balloon is filled with 38.5 L of helium gas inside a prep shed. The temperature in...
Questions

Mathematics, 30.10.2020 04:10

Mathematics, 30.10.2020 04:10


Chemistry, 30.10.2020 04:10

Mathematics, 30.10.2020 04:10






Spanish, 30.10.2020 04:10





History, 30.10.2020 04:10


Biology, 30.10.2020 04:10


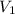 = 38.5 L,
= 38.5 L, 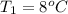
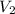 = ?,
= ?,