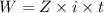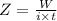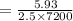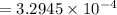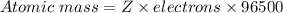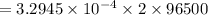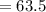A current of 2.50 amps is passed through an electrolytic cell containing a solution of M(NO3)2, where M represents an unidentified metal. The only reaction that occurs at the cathode is the deposition of the unknown metal. After 2.00 hours, 5.93 g of the metal has been deposited.
Required:
Determine the chemical symbol of the metal.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1

You know the right answer?
A current of 2.50 amps is passed through an electrolytic cell containing a solution of M(NO3)2, wher...
Questions

Arts, 26.10.2019 00:43





Chemistry, 26.10.2019 00:43

Mathematics, 26.10.2019 00:43

Biology, 26.10.2019 00:43

Mathematics, 26.10.2019 00:43





Mathematics, 26.10.2019 00:43

Health, 26.10.2019 00:43




History, 26.10.2019 00:43

 ".
".