
Chemistry, 19.05.2021 18:00 jazzyjaz2003
27.5 mL of a 0.235 M potassium hydroxide solution is required to completely react with 35.0 mL of a sulfuric acid solution. Provide the balanced chemical equation for this reaction and determine the concentration of the sulfuric acid solution.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:40
If 10.0 ml of the solution on the right are withdrawn from the 100 ml beaker and diluted again in a similar manner, what is the new concentration? m nacl
Answers: 2

Chemistry, 21.06.2019 19:00
Which of the following best explains why the end of a spoon sticking out of a cup of hot water also gets hot? question 7 options: the heat from the hot water is conducted through the spoon handle the hot water heats the air surrounding the upper part of the spoon. the hot water causes a physical change in the spoon handle. the hot water causes a chemical reaction to take place in the spoon.
Answers: 2

Chemistry, 22.06.2019 02:30
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 22.06.2019 06:00
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2
You know the right answer?
27.5 mL of a 0.235 M potassium hydroxide solution is required to completely react with 35.0 mL of a...
Questions


History, 20.03.2020 22:04

Mathematics, 20.03.2020 22:04

Mathematics, 20.03.2020 22:05






Computers and Technology, 20.03.2020 22:05




Mathematics, 20.03.2020 22:05


History, 20.03.2020 22:06

Computers and Technology, 20.03.2020 22:06


Mathematics, 20.03.2020 22:06

Mathematics, 20.03.2020 22:06

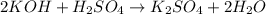 and the concentration of the sulfuric acid solution is 0.184 M.
and the concentration of the sulfuric acid solution is 0.184 M.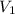 = 27.5 mL,
= 27.5 mL, 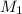 = 0.235 M
= 0.235 M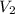 = 35.0 mL,
= 35.0 mL, 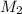 = ?
= ?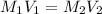
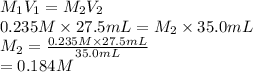

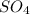 = 1O = 1
= 1O = 1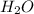 by 2. Hence, the equation can be rewritten as follows.
by 2. Hence, the equation can be rewritten as follows.

