
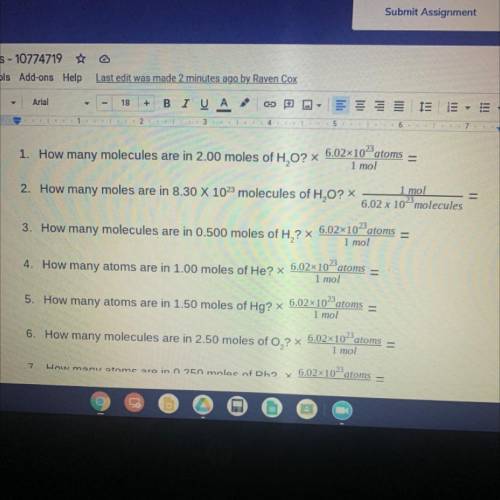
1. How many molecules are in 2.00 moles of H,0? x 6.02x10-atoms
1 mol
2. How many moles are in 8.30 X 102 molecules of H2O? *
1 mol
6.02 x 102 molecules
3. How many molecules are in 0.500 moles of H,? X
6.02x1023
atoms
1 mol
6.02x10- atoms
4. How many atoms are in 1.00 moles of He? X
1 mol
5. How many atoms are in 1.50 moles of Hg? X
6.02x10-atoms
1 mol
6. How many molecules are in 2.50 moles of O,? X
6.02x10- atoms
1 mol

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

Chemistry, 22.06.2019 20:30
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2
You know the right answer?
1. How many molecules are in 2.00 moles of H,0? x 6.02x10-atoms
1 mol
2. How many moles are i...
2. How many moles are i...
Questions

Mathematics, 16.10.2019 22:30


Physics, 16.10.2019 22:30

Biology, 16.10.2019 22:30



History, 16.10.2019 22:30

Mathematics, 16.10.2019 22:30







Geography, 16.10.2019 22:30



Mathematics, 16.10.2019 22:30


History, 16.10.2019 22:30



