
Chemistry, 19.05.2021 18:30 micheleflorack
CALCULATING HALF LIFE LAB REPORT.
I need helping filling out the two tables, making a graph and answering some questions.
The tables have not been filled out yet at all, and I haven't been able to insert the graph because of it, need some help!!
Question : scientist finds a piece of wood that is thought to be from a ancient fire Circle. They find the word that contains the amount of carbon-14 see that is approximately 1/6 of the current atmospheric 14c levels determine approximately how many years ago the tree was chopped down to be used for firewood if he started with 1 million carbon-14 atoms how many atoms of remain in the wood? 14c has a t1/2 of 5, 750 years.
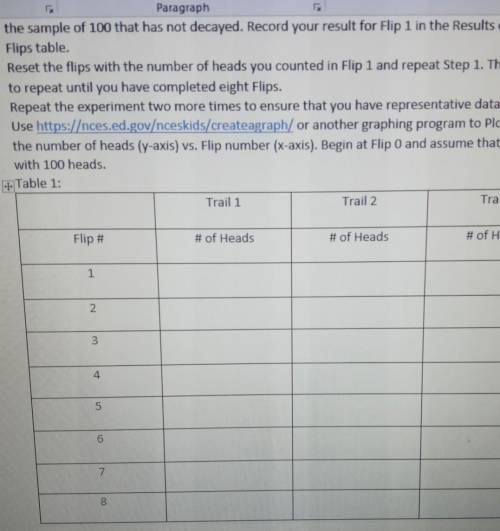

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1

Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

Chemistry, 23.06.2019 03:00
You have a sample of a metal, the sample is exactly 6.02 x 1023atom, if the sample has a mass 55.85 what metal is your sample made of?
Answers: 2
You know the right answer?
CALCULATING HALF LIFE LAB REPORT.
I need helping filling out the two tables, making a graph and ans...
Questions

History, 25.03.2020 21:51


History, 25.03.2020 21:51


Mathematics, 25.03.2020 21:52





Mathematics, 25.03.2020 21:52


Mathematics, 25.03.2020 21:52

Mathematics, 25.03.2020 21:52

Mathematics, 25.03.2020 21:52

Mathematics, 25.03.2020 21:52

History, 25.03.2020 21:52

Mathematics, 25.03.2020 21:52

Social Studies, 25.03.2020 21:52

Biology, 25.03.2020 21:52



