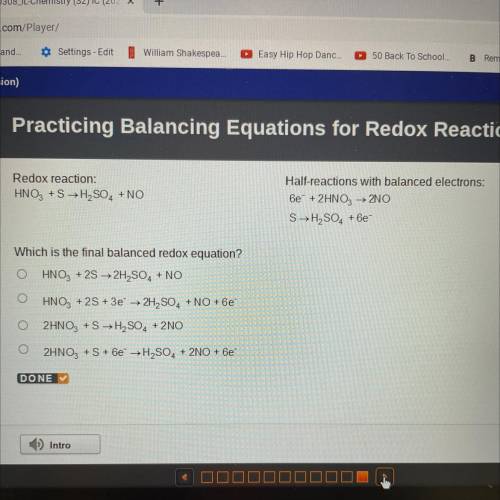
Redox reaction:
HNO3 +S → H2SO4 + NO
Half-reactions with balanced electrons:
6e + 2HNO3...

Chemistry, 19.05.2021 19:30 camballard3848
Redox reaction:
HNO3 +S → H2SO4 + NO
Half-reactions with balanced electrons:
6e + 2HNO3 → 2NO
SH2SO4 +6e
Which is the final balanced redox equation?
HNO3 + 2S → 2H2SO4 + NO
HNO3 + 2S + 3e → 2H2SO4 + NO + 6e
2HNO3 +S →H2SO4 + 2NO
2HNO3 + S + 6e → H2SO4 + 2NO + 6e

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2

Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2

Chemistry, 23.06.2019 01:00
What type of chemical bond is formed between two atoms of bromine 1. metallic 2. hydrogen 3. ionic 4. covalent
Answers: 1
You know the right answer?
Questions






Mathematics, 11.12.2019 06:31

History, 11.12.2019 06:31



Chemistry, 11.12.2019 06:31





Mathematics, 11.12.2019 06:31

Mathematics, 11.12.2019 06:31




History, 11.12.2019 06:31



