
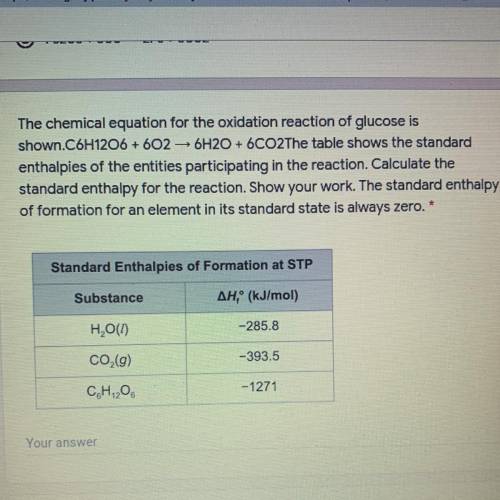
PLEASE HELP ME WITH THIS ! The chemical equation for the oxidation reaction of glucose is
shown. C6H12O6 + 602 6H2O + 6CO2The table shows the standard
enthalpies of the entities participating in the reaction. Calculate the
standard enthalpy for the reaction. Show your work. The standard enthalpy
of formation for an element in its standard state is always zero.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1

Chemistry, 22.06.2019 02:00
If you add 10ml of hot water to 10ml of cold water and the change in tempature 8°c calculate how much energy is gained by the cold water
Answers: 1

Chemistry, 22.06.2019 03:00
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3
You know the right answer?
PLEASE HELP ME WITH THIS ! The chemical equation for the oxidation reaction of glucose is
shown. C6...
Questions


History, 28.09.2019 02:30




Health, 28.09.2019 02:30


Mathematics, 28.09.2019 02:30

English, 28.09.2019 02:30

Health, 28.09.2019 02:30

History, 28.09.2019 02:30

Physics, 28.09.2019 02:30

Mathematics, 28.09.2019 02:30

Business, 28.09.2019 02:30

Computers and Technology, 28.09.2019 02:30

Mathematics, 28.09.2019 02:30



English, 28.09.2019 02:30

Biology, 28.09.2019 02:30



