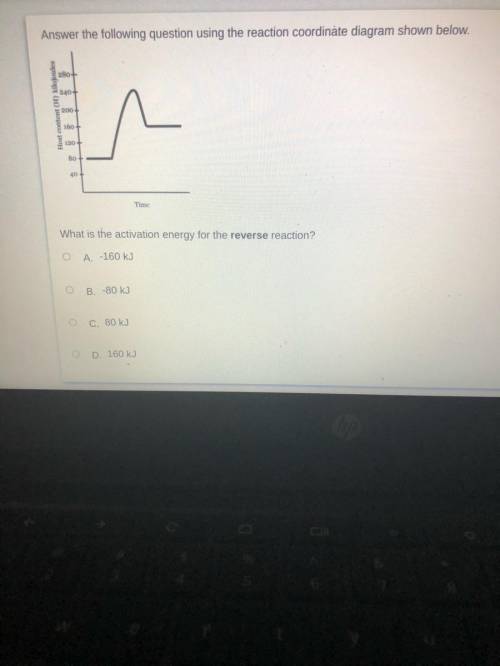
A.-160kJ
B.-80kJ
C.80kJ
D.160.kJ
...

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 23.06.2019 01:00
Wind and moving water provide energy. chemical mechanical thermal none of the above
Answers: 1

Chemistry, 23.06.2019 05:40
Convert a speed of 201 cm/s to units of inches per minute. also, show the unit analysis by dragging components into the unit‑factor slots.
Answers: 1
You know the right answer?
Questions

Health, 01.07.2019 10:30



Health, 01.07.2019 10:30


Mathematics, 01.07.2019 10:30


Computers and Technology, 01.07.2019 10:30




History, 01.07.2019 10:30




History, 01.07.2019 10:30

Biology, 01.07.2019 10:30


Health, 01.07.2019 10:30

Mathematics, 01.07.2019 10:30