Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1

Chemistry, 22.06.2019 01:30
100 points answer quick the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 06:30
The minerals found in bones are deposited by living cells called
Answers: 1
You know the right answer?
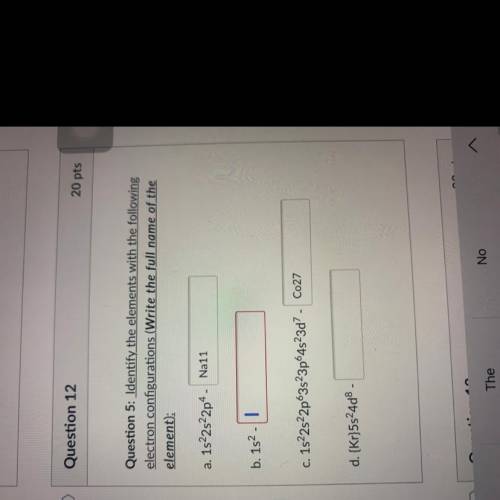
Question 5: Identify the elements with the following
electron configurations (Write the full name o...
Questions




Mathematics, 21.12.2020 16:20

Biology, 21.12.2020 16:20


English, 21.12.2020 16:20








Mathematics, 21.12.2020 16:20



Computers and Technology, 21.12.2020 16:20


SAT, 21.12.2020 16:20