
Chemistry, 20.05.2021 18:40 Chewychipsx4023
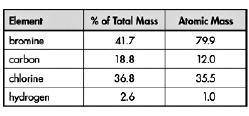
In order to identify an unknown compound found at an environmental cleanup site, an analytical chemist determines the percent composition of the compound. The results are shown in the table below.
What is the empirical formula of the compound?
a
C3H5Cl2Br
b
CHClBr
c
C4HCl5Br10
d
C3H5ClBr2

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1

You know the right answer?
In order to identify an unknown compound found at an environmental cleanup site, an analytical chemi...
Questions


Arts, 21.11.2020 02:50




Mathematics, 21.11.2020 02:50


Mathematics, 21.11.2020 02:50

English, 21.11.2020 02:50

Social Studies, 21.11.2020 02:50

Mathematics, 21.11.2020 02:50



Mathematics, 21.11.2020 02:50


History, 21.11.2020 02:50


Computers and Technology, 21.11.2020 02:50




