Chemistry, 20.05.2021 19:40 nicholasferrell
The initial temperature of the water in a constant-pressure calorimeter is
14°C. A reaction takes place in the calorimeter, and the temperature rises
to 87°C. The calorimeter contains 254 g of water, which has a specific heat
of 4.18 J/(g.°C). Calculate the enthalpy change during this reaction. *

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Describe the chemical reaction based on the chemical equation below. also, explain whether the equation is balanced.
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3

Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1

Chemistry, 23.06.2019 00:00
How many peaks will be present in a mass spectrum for brcl?
Answers: 1
You know the right answer?
The initial temperature of the water in a constant-pressure calorimeter is
14°C. A reaction takes p...
Questions

Physics, 24.07.2019 01:30


Chemistry, 24.07.2019 01:30

Mathematics, 24.07.2019 01:30


Mathematics, 24.07.2019 01:30

History, 24.07.2019 01:30





History, 24.07.2019 01:30


Mathematics, 24.07.2019 01:30


Mathematics, 24.07.2019 01:30

History, 24.07.2019 01:30


History, 24.07.2019 01:30

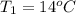 ,
, 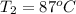
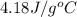
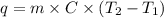
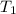 = initial temperature
= initial temperature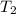 = final temperature
= final temperature