2 H2 + O2
2 H2O
Use the above equation to solve for the following: (Show your work for the ma...

Chemistry, 20.05.2021 20:20 irelandcrawford5469
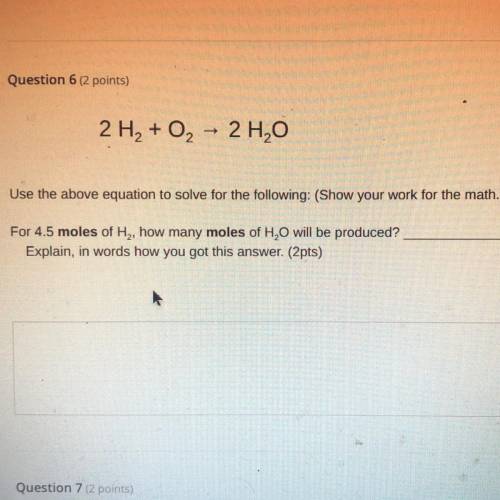
2 H2 + O2
2 H2O
Use the above equation to solve for the following: (Show your work for the math.)
For 4.5 moles of H, how many moles of H, O will be produced?
Explain, in words how you got this answer. (2pts)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2


Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1

Chemistry, 22.06.2019 22:00
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1
You know the right answer?
Questions

Physics, 14.07.2020 01:01

Mathematics, 14.07.2020 01:01



Mathematics, 14.07.2020 01:01

English, 14.07.2020 01:01

Mathematics, 14.07.2020 01:01

Mathematics, 14.07.2020 01:01

Mathematics, 14.07.2020 01:01

Mathematics, 14.07.2020 01:01

English, 14.07.2020 01:01


Social Studies, 14.07.2020 01:01




Spanish, 14.07.2020 01:01


History, 14.07.2020 01:01

Mathematics, 14.07.2020 01:01



