Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 17:20
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2
You know the right answer?
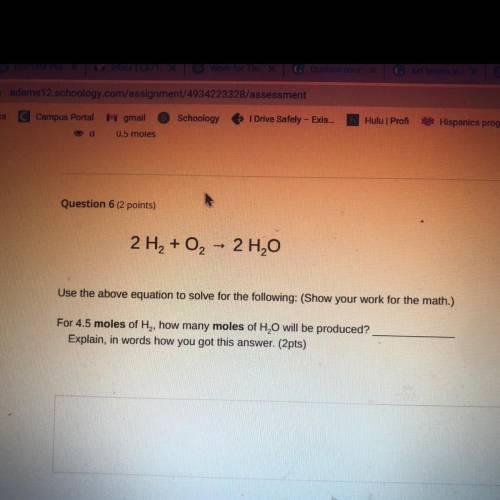
2 H2 + O2 + 2 H2O
Use the above equation to solve for the following: (Show your work for the math.)...
Questions

English, 25.06.2019 15:00

English, 25.06.2019 15:00

Mathematics, 25.06.2019 15:00







History, 25.06.2019 15:00



Mathematics, 25.06.2019 15:00


Mathematics, 25.06.2019 15:00

Social Studies, 25.06.2019 15:00



Biology, 25.06.2019 15:00

Mathematics, 25.06.2019 15:00