The information below describes a redox reaction.
Ag+ (aq) + Al(s) — Ag(s) + A13+ (aq)
Ag+ (a...

Chemistry, 20.05.2021 22:40 lilpeepxliltracy
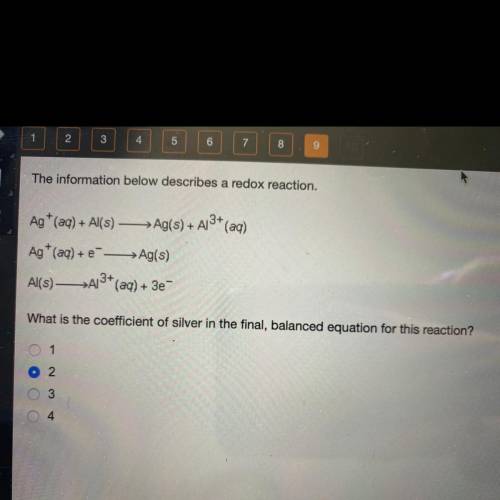
The information below describes a redox reaction.
Ag+ (aq) + Al(s) — Ag(s) + A13+ (aq)
Ag+ (aq) + --> Ag(s)
Al(s)—>A13+ (aq) + 3e-
What is the coefficient of silver in the final, balanced equation for this reaction?
1
2
3
4

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 14:30
The length of a vector arrow represents its magnitude and the point represents its direction true or false apex
Answers: 3


Chemistry, 22.06.2019 04:00
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1
You know the right answer?
Questions


History, 11.02.2021 05:10


Mathematics, 11.02.2021 05:10

English, 11.02.2021 05:10

Mathematics, 11.02.2021 05:10



Mathematics, 11.02.2021 05:10


Biology, 11.02.2021 05:10

Mathematics, 11.02.2021 05:10

Mathematics, 11.02.2021 05:10

Computers and Technology, 11.02.2021 05:10


Mathematics, 11.02.2021 05:10

History, 11.02.2021 05:10


Mathematics, 11.02.2021 05:10

History, 11.02.2021 05:10



