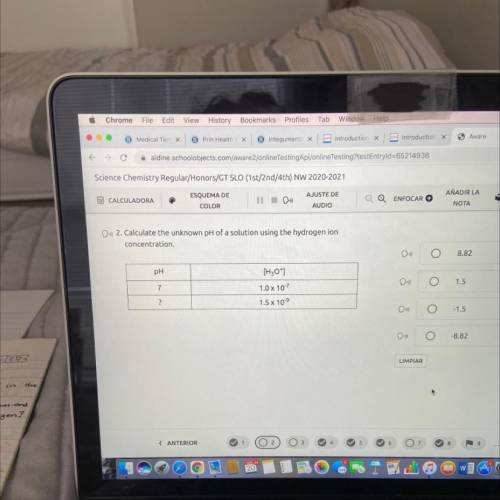
Calculate the unknown pH of a solution using the hydrogen ion
concentration.
A) 8.82
B)...

Chemistry, 21.05.2021 01:20 joeykyle05
Calculate the unknown pH of a solution using the hydrogen ion
concentration.
A) 8.82
B) 1.5
C) -1.5
D) -8.82

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

Chemistry, 22.06.2019 21:30
Plzz a sample of table sugar (sucrose, c12h22o11) has a mass of 7.801 g. ● a) calculate the number of moles of c12h22o11 in the sample b) calculate the number of moles of each element in c12h22o11 (number of moles of c, number of moles of h & number of moles of o) in the sample. (use your answer from part a as your starting point.) show your work and highlight your final answer. calculate the number of atoms of each element in c12h22o11 (number of atoms of c, number of atoms of h & number of atoms of o) in the sample. (use your answers from part b as your starting for each element.) show your work and highlight your final answer.
Answers: 1
You know the right answer?
Questions


Social Studies, 17.05.2021 14:00

Mathematics, 17.05.2021 14:00

English, 17.05.2021 14:00

Geography, 17.05.2021 14:00

Mathematics, 17.05.2021 14:00


Physics, 17.05.2021 14:00

Mathematics, 17.05.2021 14:00

Geography, 17.05.2021 14:00

Mathematics, 17.05.2021 14:00


Mathematics, 17.05.2021 14:00


Mathematics, 17.05.2021 14:00

Mathematics, 17.05.2021 14:00




Mathematics, 17.05.2021 14:00



