
Chemistry, 21.05.2021 01:50 starburst2005
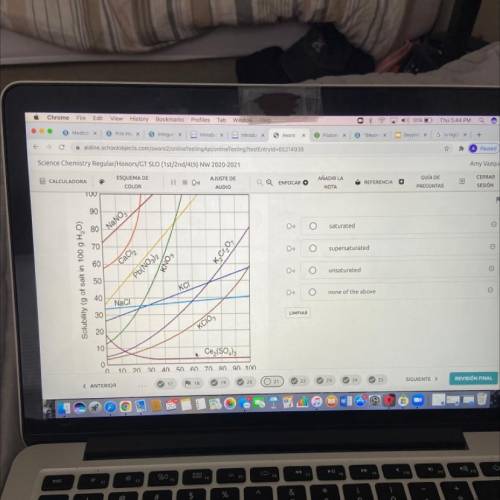
A student prepares a solution of potassium nitrate KNO3 containing 95 g dissolved at 40degrees Celsius. This solution is
A)saturated
B)supersaturated
C) unsaturated
D)none of the above

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:30
Five students had to answer the question how are elements arranged in a periodic tabledamon: i think the elements are arranged by increasing massflo: i think the elements are arranged according to their properties sienna: i think the elements are arranged by when their discovers kyle: i think the elements are arranged according to how common they areglenda: i don't agree with any of themwho is right
Answers: 1

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 22.06.2019 13:30
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3
You know the right answer?
A student prepares a solution of potassium nitrate KNO3 containing 95 g dissolved at 40degrees Celsi...
Questions

Mathematics, 23.04.2020 12:29


English, 23.04.2020 12:29

Mathematics, 23.04.2020 12:29

English, 23.04.2020 12:29


Mathematics, 23.04.2020 12:29


Biology, 23.04.2020 12:29

Mathematics, 23.04.2020 12:29

Mathematics, 23.04.2020 12:29



Mathematics, 23.04.2020 12:29

History, 23.04.2020 12:29

Biology, 23.04.2020 12:29

Mathematics, 23.04.2020 12:29





