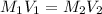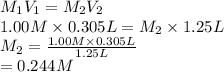Chemistry, 21.05.2021 05:40 kjmccarty02
The student then takes a 1.00M stock solution of table sugar (sucrose C12H22O11) and mixes 0.305L of stock solution with additional distilled water to create a dilute solution with a total volume of 1.25L. Explain how the student can determine the molarity of the resulting solution. Show a valid calculation for the final molarity. PLS HELP

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2

Chemistry, 23.06.2019 10:20
Based on the equation, how many grams of br2 are required to react completely with 29.2 grams of alcl3? alcl3 + br2 → albr3 + cl2 48.7 grams 52.6 grams 56.7 grams 61.3 grams
Answers: 3
You know the right answer?
The student then takes a 1.00M stock solution of table sugar (sucrose C12H22O11) and mixes 0.305L of...
Questions




Mathematics, 15.04.2020 00:54




English, 15.04.2020 00:55





English, 15.04.2020 00:55

Mathematics, 15.04.2020 00:55

Computers and Technology, 15.04.2020 00:55




Chemistry, 15.04.2020 00:55

Arts, 15.04.2020 00:55

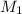 = 1.00 M,
= 1.00 M, 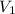 = 0.305 L
= 0.305 L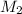 = ?,
= ?, 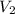 = 1.25 L
= 1.25 L