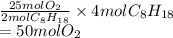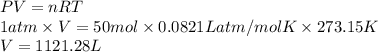Chemistry, 21.05.2021 16:30 kirstenb278
If 4.00 moles of gasoline are burned according to the chemical
reaction below, what volume of oxygen at STP is needed for complete
combustion?
2C2H18(I) + 25O2(g) → 16CO2(g) +18H2O(g)
Need done in 20 min if anyone could I help I’ll mark you as a brainiest!!

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3
You know the right answer?
If 4.00 moles of gasoline are burned according to the chemical
reaction below, what volume of oxyge...
Questions



English, 29.04.2021 20:20


Mathematics, 29.04.2021 20:20


Mathematics, 29.04.2021 20:20



Mathematics, 29.04.2021 20:20

Mathematics, 29.04.2021 20:20

Mathematics, 29.04.2021 20:20


Mathematics, 29.04.2021 20:20

Geography, 29.04.2021 20:20



Mathematics, 29.04.2021 20:20


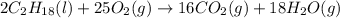
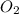 . Hence, moles of oxygen required to react with 4 moles of gasoline are calculated as follows.
. Hence, moles of oxygen required to react with 4 moles of gasoline are calculated as follows.