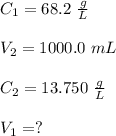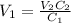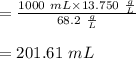Chemistry, 21.05.2021 17:50 aderahd7352
A stock solution has a concentration of 68.2 g/L. A 13.750 g/L solution is required. If you use a 1000.0 mL volumetric flask for the dilution, what volume (in ml) needs to be taken from the stock solution? Give your answer to three significant figures.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:10
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1

Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3
You know the right answer?
A stock solution has a concentration of 68.2 g/L. A 13.750 g/L solution is required. If you use a 10...
Questions














Social Studies, 23.01.2020 17:31