
Chemistry, 21.05.2021 17:50 jmt13happy
In each pair of compounds, pick the one with the higher vapor pressure at a given temperature. Explain your reasoning.
a. CH4 or CH3CI
b. CH3CH2CH2OH or CH3OH
c. CH3OH or H2CO

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
When curium-242 is bombarded with an alpha particle, two products are formed, one of which is a nudge on. what is the other product
Answers: 3

Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2
You know the right answer?
In each pair of compounds, pick the one with the higher vapor pressure at a given temperature. Expla...
Questions







Mathematics, 09.08.2019 21:30


History, 09.08.2019 21:30




Mathematics, 09.08.2019 21:30







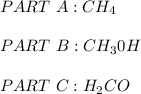
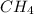 because it is non-polar, while
because it is non-polar, while 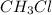 is polar.
is polar.  .
.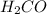 does not show hydrogen due to the increased vapor pressure
does not show hydrogen due to the increased vapor pressure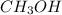 bonding.
bonding.


