
Chemistry, 21.05.2021 19:00 logan541972
You had a 69.24 g piece of unknown metal and placed it into hot water at 103°C. You then performed the same experiment as done in class (specific heat of a metal lab) using a Styrofoam cup calorimeter (filled with 75.0 mL of water) and you found that the temperature of the water in the cup rose from 23.0˚C to 27.6˚C, what would the metal’s specific heat be? Identify the metal from the table. (The specific heat of water is 4.184 J/g) I need work to be shown
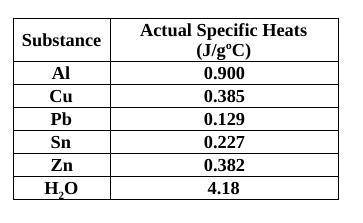

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:50
Which statement describes how phase changes can be diagrammed as a substance is heated? the phase is on the y-axis and the temperature is on the x-axis. the temperature is on the y-axis and the phase is on the x-axis. the time is on the y-axis and the temperature is on the x-axis. the temperature is on the y-axis and the time is on the x-axis.
Answers: 1

Chemistry, 22.06.2019 00:30
13. calculate the initial concentration (before precipitation) of carbonate ions after the addition of each 0.05 ml of solution b to the 1.00 l beaker of solution a. divide the work among group members and write the answers in the table in model 3. assume the volume change as solution b is added is negligible. 14. notice the initial concentrations of zn2+ - and cu2+ in the table in model 3. a. explain how these were obtained from the data in model 2. b. as solution b is added and precipitates form, do these initial concentrations change? 15. use the data in model 2 to indicate the presence of precipitate (either znco3 or cuco3) after each 0.05 ml addition of solution b in model 3. 16. use the initial concentrations of carbonate ions and zinc ions to calculate the reaction quotient, qsp for the zinc carbonate scenarios in model 3. divide the work among group members and write the answers in the table in model 3. 17. use the initial concentrations of carbonate ion and copper(ii) ions to calculate the qsp for the copper(ii) carbonate scenarios in model 3. divide the work among group members and write the answers in the table in model 3.
Answers: 3


Chemistry, 23.06.2019 01:00
Which fossil fuel is mainly used for heating and cooking? a. electricity b. coal c. petroleum d. natural gas
Answers: 2
You know the right answer?
You had a 69.24 g piece of unknown metal and placed it into hot water at 103°C. You then performed t...
Questions

Chemistry, 18.09.2020 07:01

Mathematics, 18.09.2020 07:01

Mathematics, 18.09.2020 07:01

Physics, 18.09.2020 07:01

Mathematics, 18.09.2020 07:01

Mathematics, 18.09.2020 07:01

Mathematics, 18.09.2020 07:01

Mathematics, 18.09.2020 07:01

Mathematics, 18.09.2020 07:01

Mathematics, 18.09.2020 07:01

Mathematics, 18.09.2020 07:01

Mathematics, 18.09.2020 07:01

Physics, 18.09.2020 07:01

Mathematics, 18.09.2020 07:01

Mathematics, 18.09.2020 07:01

Mathematics, 18.09.2020 07:01

Mathematics, 18.09.2020 07:01

Physics, 18.09.2020 07:01

Mathematics, 18.09.2020 07:01

English, 18.09.2020 07:01



