
Chemistry, 21.05.2021 19:40 twinkieslayer
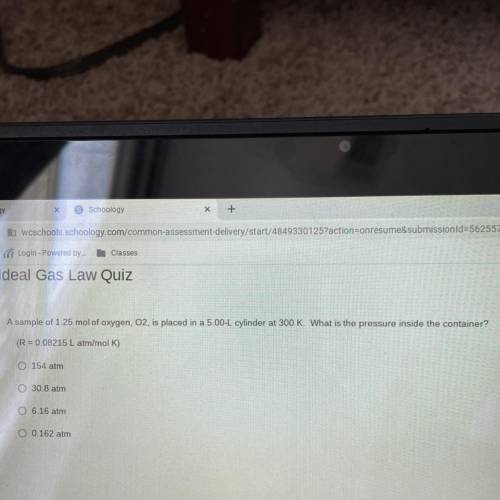
A sample of 1.25 mol of oxygen, 02, is placed in a 5.00-L cylinder at 300 K. What is the pressure inside the container?
(R = 0.08215 L atm/mol K)
154 atm
30.8 atm
6.16 atm
0.162 atm

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:00
How did planetesmals form planets? a. they broke apart into smaller chunks.b. they collided and stuck together.c. they cooled and pulled ice together.d. they began to rotate.
Answers: 1

Chemistry, 22.06.2019 02:00
What is the maximum number of electrons that an atomic orbital can contain?
Answers: 1

Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 07:00
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1
You know the right answer?
A sample of 1.25 mol of oxygen, 02, is placed in a 5.00-L cylinder at 300 K. What is the pressure in...
Questions



Mathematics, 10.04.2021 02:50

Mathematics, 10.04.2021 02:50

Mathematics, 10.04.2021 02:50



Advanced Placement (AP), 10.04.2021 02:50




History, 10.04.2021 02:50

Social Studies, 10.04.2021 02:50



Mathematics, 10.04.2021 02:50



Social Studies, 10.04.2021 02:50



