Write the formula for the for the following reaction:
Use:
...

Chemistry, 21.05.2021 20:00 nayelieangueira
Write the formula for the 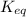 for the following reaction:
for the following reaction:
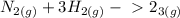
Use:
![K_{eq} =\frac{[C]^{c} [D]^d }{[A]^a[B]^b}](/tpl/images/2369/8760/65bf2.png)

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
In order to calculate the amount of heat transferred you must know the __ and specific heat of the material, as well as the change in temperature. a. volume b. density c. mass d. enthalpy
Answers: 1

Chemistry, 22.06.2019 11:00
Surface currents are caused by blank space . question 14 options: surface currents are caused by? differences in water temperature high salinity differences in density wind forces
Answers: 1

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2

Chemistry, 22.06.2019 22:00
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1
You know the right answer?
Questions

Social Studies, 16.04.2020 04:00

Mathematics, 16.04.2020 04:00




Physics, 16.04.2020 04:00

Mathematics, 16.04.2020 04:00

Mathematics, 16.04.2020 04:00


Spanish, 16.04.2020 04:00

Mathematics, 16.04.2020 04:00



Mathematics, 16.04.2020 04:00






Mathematics, 16.04.2020 04:01



