
Chemistry, 21.05.2021 20:10 dari122223
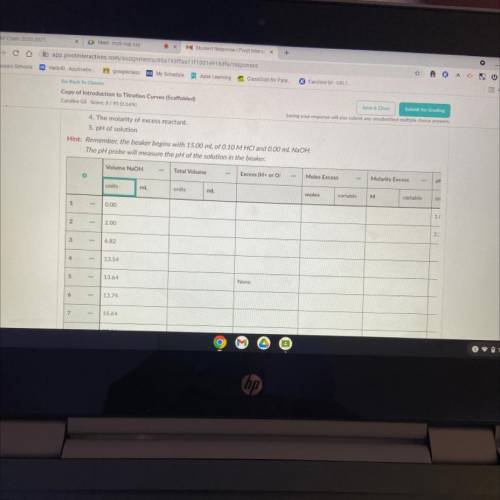
6. Now that you've predicted the equivalence point, let's predict several other points on the titration curve.
Perform calculations to predict the pH at the following points in the titration.
• 0.00 ml NaOH added
• 2.00 ml NaOH
• Half of the equivalence point volume
• 0.10 ml before the equivalence point
• Equivalence Point
• 0.10 mL after equivalence point
• 2.00 mL after the equivalence point
• 10.00 mL after equivalence point

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 7.3 × 106 m/s. show your work. note: h = plank's constant (6.62607 x 10-34 j s)
Answers: 1

Chemistry, 23.06.2019 11:00
The standard emf for the cell using the overall cell reaction below is +2.20 v: 2al(s) + 3i2(s) → 2ai3+(aq) + 6i-(aq) the emf generated by the cell when [ai3+] = 3.5 × 10-3 m and [i-] = 0.015 m is v. the standard emf for the cell using the overall cell reaction below is +2.20 v: 2al(s) + 3i2(s) 2ai3+(aq) + 6i-(aq) the emf generated by the cell when [ai3+] = 3.5 × 10-3 m and [i-] = 0.015 m is v. 2.36 2.24 2.21 2.51 2.04
Answers: 2
You know the right answer?
6. Now that you've predicted the equivalence point, let's predict several other points on the titrat...
Questions

Mathematics, 03.03.2021 22:40

English, 03.03.2021 22:40



Computers and Technology, 03.03.2021 22:50


French, 03.03.2021 22:50

Chemistry, 03.03.2021 22:50


Biology, 03.03.2021 22:50

Mathematics, 03.03.2021 22:50

Mathematics, 03.03.2021 22:50

English, 03.03.2021 22:50


Arts, 03.03.2021 22:50

Mathematics, 03.03.2021 22:50

Mathematics, 03.03.2021 22:50





