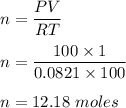Chemistry, 21.05.2021 21:20 tabathahasaunicorn1
How many moles of gas are in a 1.0 liter canister if the temperature of the canister is 100 K and the pressure is 100 atmospheres? R = 0.0821 L*atm/mol*K (Celsius + 273 = Kelvin)

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1
You know the right answer?
How many moles of gas are in a 1.0 liter canister if the temperature of the canister is 100 K and th...
Questions

Mathematics, 11.03.2020 04:37



Mathematics, 11.03.2020 04:37


Chemistry, 11.03.2020 04:37

Mathematics, 11.03.2020 04:37



Physics, 11.03.2020 04:37

Mathematics, 11.03.2020 04:37



Mathematics, 11.03.2020 04:37

History, 11.03.2020 04:37