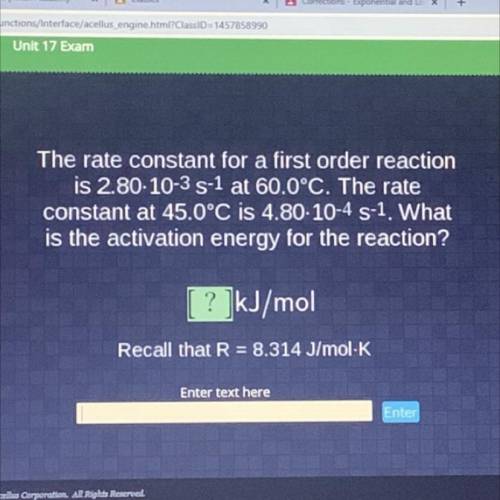
The rate constant for a first order reaction
is 2.80-10-35-1 at 60.0°C. The rate
constant at...


Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:30
What volume of a 2.00 m stock solution of naoh is needed to prepare 150. ml of 0.40 m solution?
Answers: 2

Chemistry, 22.06.2019 03:00
Which of these would be caused by a chemical change? a) the formation of lava. b) sedimantary rock layering over time. c) metamorphic rock forming from igneous. d) metamorphic rock eroding to form sedimentary rock.
Answers: 3

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1
You know the right answer?
Questions


Mathematics, 28.12.2019 00:31

Computers and Technology, 28.12.2019 00:31


Geography, 28.12.2019 00:31

English, 28.12.2019 00:31


History, 28.12.2019 00:31

Physics, 28.12.2019 00:31

Mathematics, 28.12.2019 00:31

Mathematics, 28.12.2019 00:31

English, 28.12.2019 00:31



History, 28.12.2019 00:31


Mathematics, 28.12.2019 00:31

Biology, 28.12.2019 00:31


Health, 28.12.2019 00:31