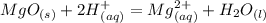Chemistry, 22.05.2021 18:10 mistycascaden
What is the ionic equation for this reaction:
MgO (s) + 2HCl (aq) = MgCl2 (aq) + H2O (l)
Please let me know how you worked it out, thankyou!!

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 14:30
Janel’s class studied properties of matter and how matter can change. janel decided she would do an experiment mixing baking soda and vinegar.question: describe the properties of baking soda and vinegar, and explain the changes that janel should see when she mixes the two types of matter. •first, identify the physical state of matter of baking soda. describe another property of baking soda. •next, identify the physical state of matter of vinegar. describe another property of vinegar. •then, explain what janel should see when she mixes the baking soda and vinegar. •describe the states of matter of the new materials that are formed. •explain how janel can be certain a change has occurred. me
Answers: 3

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 23:00
What element has similar physical and chemical properties as boron.
Answers: 1
You know the right answer?
What is the ionic equation for this reaction:
MgO (s) + 2HCl (aq) = MgCl2 (aq) + H2O (l)
Questions


Mathematics, 20.02.2020 06:04




Mathematics, 20.02.2020 06:04


Social Studies, 20.02.2020 06:04





Mathematics, 20.02.2020 06:05


Mathematics, 20.02.2020 06:05

Mathematics, 20.02.2020 06:05


French, 20.02.2020 06:05

English, 20.02.2020 06:05