
Chemistry, 23.05.2021 14:00 zahriaarana
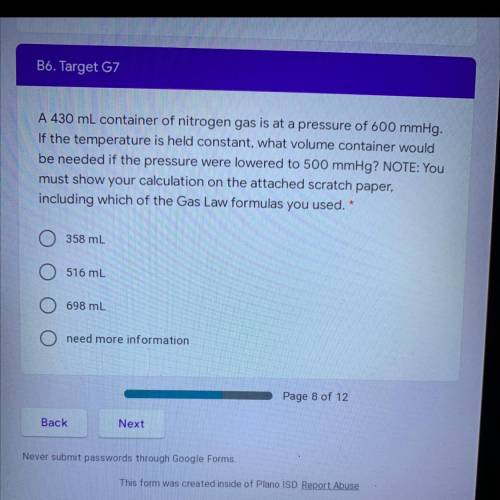
A 430 mL container of nitrogen gas is at a pressure of 600 mmHg.
If the temperature is held constant, what volume container would
be needed if the pressure were lowered to 500 mmHg? NOTE: You
must show your calculation on the attached scratch paper, including which of the Gas Law formulas you used.
A. 358 mL
B. 516 mL
C. 698 mL
D. need more information
(Show your work please)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Aphysical reaction is a process in which one or more reactants change into one or more products with different properties. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1


Chemistry, 22.06.2019 20:00
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3
You know the right answer?
A 430 mL container of nitrogen gas is at a pressure of 600 mmHg.
If the temperature is held constan...
Questions








Mathematics, 08.09.2020 23:01

Mathematics, 08.09.2020 23:01



History, 08.09.2020 23:01

English, 08.09.2020 23:01

History, 08.09.2020 23:01

Mathematics, 08.09.2020 23:01








