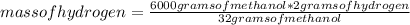Chemistry, 24.05.2021 07:30 bobiscool3698
Determine what mass of carbon monoxide and what mass of hydrogen are required to form 6.0 kg of methanol by the reaction CO(g) + 2H2(g) -> C H3OH(l)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1
You know the right answer?
Determine what mass of carbon monoxide and what mass of hydrogen are required to form 6.0 kg of meth...
Questions





Mathematics, 15.04.2021 22:30




Computers and Technology, 15.04.2021 22:30

Social Studies, 15.04.2021 22:30

History, 15.04.2021 22:30

Mathematics, 15.04.2021 22:30

Mathematics, 15.04.2021 22:30

Mathematics, 15.04.2021 22:30

English, 15.04.2021 22:30



Health, 15.04.2021 22:30