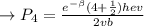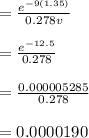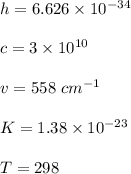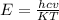Chemistry, 24.05.2021 14:00 romanlittlewood
The first excited vibrational energy level of diatomic chlo- rine (Cl2) is 558 cm^-1 above the ground state. Wave- numbers, the units in which vibrational frequencies are usually recorded, are effectively units of energy, with 1 cm ^-1 = 1.986445 X 10^-23 J. If every vibrational energy level is equally spaced, and has a degeneracy of 1, sum over the lowest 4 vibrational levels to obtain a vibrational partition function for chlorine.
Required:
Determine the populations of each of the four levels at 298 K and the average molar vibrational energy (Em. vib) for chlorine at 298 K.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 21:00
Need what is special about water as a compound? how does water regulate climate? what drives water evaporation? why is the water vapor fresh water when it rises from the ocean? why might freshwater in the form of snow take longer to enter the water cycle again than liquid precipitation? what is an aquifer? what role do people play in the water cycle? plz just answer as many as you can ! thx if you !
Answers: 1

Chemistry, 23.06.2019 06:00
Jenny wants to test the electrical conductivity of two substances dissolved in water. she is preparing the containers for the experiment. which factor is most important for her to control?
Answers: 1

Chemistry, 23.06.2019 07:00
How does science use models to gain a better understanding of concepts?
Answers: 1

Chemistry, 23.06.2019 07:30
Type the letter that represents the correct location for each particle type below. the neutron is found at __ the electron is found at __ the proton is found at __
Answers: 1
You know the right answer?
The first excited vibrational energy level of diatomic chlo- rine (Cl2) is 558 cm^-1 above the groun...
Questions


Mathematics, 01.09.2020 01:01

Social Studies, 01.09.2020 01:01

Mathematics, 01.09.2020 01:01


Biology, 01.09.2020 01:01

Mathematics, 01.09.2020 01:01


English, 01.09.2020 01:01

History, 01.09.2020 01:01


Mathematics, 01.09.2020 01:01

Chemistry, 01.09.2020 01:01


Mathematics, 01.09.2020 01:01


Mathematics, 01.09.2020 01:01

Mathematics, 01.09.2020 01:01

Mathematics, 01.09.2020 01:01

History, 01.09.2020 01:01