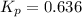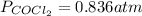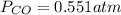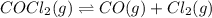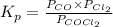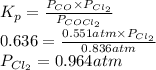The equilibrium constant, Kp, for the following reaction is 0.636 at 600K.
COCl2(g) <=> CO(g) + Cl2(g)
If an equilibrium mixture of the three gases in a 16.9 L container at 600K contains COCl2 at a pressure of 0.836 atm and CO at a pressure of 0.551 atm, the equilibrium partial pressure of Cl2 is atm.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 23:10
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium.b)heavier than helium.c)the same weight as helium.d)dependent on the element that reacted with carbon.
Answers: 3

Chemistry, 23.06.2019 01:30
Polar bears give birth and hunt on sea ice. which of the following would polar bears survive during the melting of arctic ice? growing another layer of fur during summer migrate inland to search for different food sources staying put until the ice refreezes sticking to the usual diet of seals
Answers: 1

Chemistry, 23.06.2019 08:00
At 35.0°c and 3.00 atm pressure, a gas has a volume of 1.40 l. what pressure does the gas have at 0.00°c and a volume of 0.950 l? which equation should you use? p2= p1v1t2/t1v2what is the pressure of the gas? 3.92 atm these are the answers
Answers: 1
You know the right answer?
The equilibrium constant, Kp, for the following reaction is 0.636 at 600K.
COCl2(g) <=> CO(g)...
Questions

Mathematics, 31.01.2020 14:47


Mathematics, 31.01.2020 14:47



Geography, 31.01.2020 14:47

History, 31.01.2020 14:47



Mathematics, 31.01.2020 14:47

Mathematics, 31.01.2020 14:47


Mathematics, 31.01.2020 14:47


Mathematics, 31.01.2020 14:47


Mathematics, 31.01.2020 14:47

English, 31.01.2020 14:47


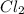 is 0.964 atm.
is 0.964 atm.