
Chemistry, 24.05.2021 21:40 jadejordan8888
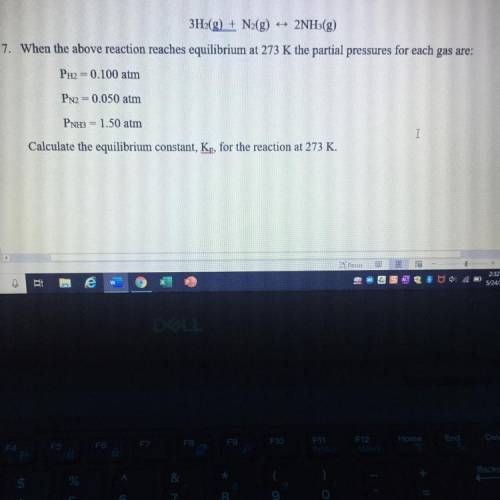
3H2(g) + N2(g) 2NH3(g)
When the above reaction reaches equilibrium at 273 K the partial pressures for each gas are:
PH2 = 0.100 atm
PN2 = 0.050 atm
PNH3 = 1.50 atm
Calculate the equilibrium constant, Kp, for the reaction at 273 K.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 13:00
What type of reaction is represented by the following example? 2co2 (g) + 4h2o (l) + 1452 kj 2ch3oh (l) (g) + 3o2 (g) exothermic endothermic
Answers: 1

Chemistry, 21.06.2019 15:40
Describe in detail the melting point behavior of the 80: 20 benzoic acid-mandelic acid mixture
Answers: 3

Chemistry, 22.06.2019 07:30
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1
You know the right answer?
3H2(g) + N2(g) 2NH3(g)
When the above reaction reaches equilibrium at 273 K the partial pressures f...
Questions








Mathematics, 17.06.2021 21:20




Mathematics, 17.06.2021 21:20



Mathematics, 17.06.2021 21:20



Mathematics, 17.06.2021 21:20

Social Studies, 17.06.2021 21:20



