
Chemistry, 24.05.2021 21:50 jasonkindred21
NEED HELP ASSP!!
(If you could show all the steps or work to get the answers that would be greatly appreciated)
62. A compound was found to contain 49.98 g of carbon and 10.47 g of hydrogen. The molar
mass of the compound is 58.12 g/mol. Determine the molecular formula.
63. A colorless liquid
composed of 46.68% nitrogen and 53.32% oxygen has a molar mass of
60.01 g/mol. What is the molecular formula?
64. When an oxide of potassium is decomposed, 19.55 g of K and 4.00 g of O are obtained.
What is the empirical formula for the compound?
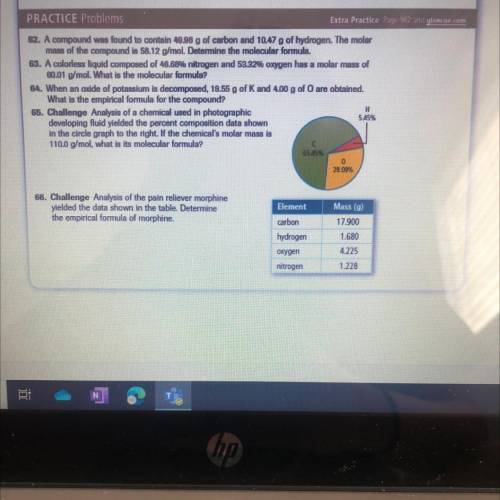
65. Challenge Analysis of a chemical used in photographic
developing fluid yielded the percent composition data shown
in the circle graph to the right. If the chemical's molar mass is
110.0 g/mol, what is its molecular formula?
66. Challenge Analysis of the pain reliever morphine
yielded the data shown in the table. Determine
the empirical formula of morphine.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3

Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1
You know the right answer?
NEED HELP ASSP!!
(If you could show all the steps or work to get the answers that would be greatly...
Questions


History, 20.03.2020 21:27



Mathematics, 20.03.2020 21:27





History, 20.03.2020 21:28

Computers and Technology, 20.03.2020 21:28


Mathematics, 20.03.2020 21:29






History, 20.03.2020 21:29



