(Can you please show all the work that was needed for the problems thank youu sm)
15. On...

Chemistry, 24.05.2021 22:10 Flowershere121
(Can you please show all the work that was needed for the problems thank youu sm)
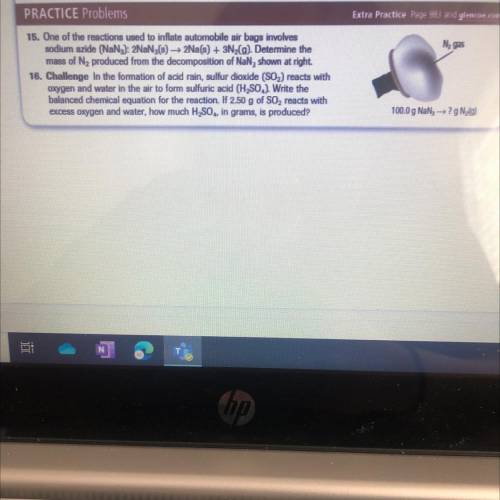
15. One of the reactions used to inflate automobile air bags involves
sodium azide (NaN3): 2NaN3(s) —> 2Na(s) + 3N2(g). Determine the
mass of N2 produced from the decomposition of NaN3 shown at right.
16. Challenge in the formation of acid rain, sulfur dioxide (S02) reacts with
oxygen and water in the air to form sulfuric acid (H2SO4) Write the
balanced chemical equation for the reaction. If 2.50 g of SO2 reacts with
excess oxygen and water, how much H2SO4 in grams, is produced?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:00
If the particles in a sample of matter have an orderly arrangement and move only in place, the sample is a
Answers: 1

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3


Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2
You know the right answer?
Questions

SAT, 09.10.2021 21:40

Computers and Technology, 09.10.2021 21:40


Mathematics, 09.10.2021 21:40



Mathematics, 09.10.2021 21:40




Biology, 09.10.2021 21:40





Mathematics, 09.10.2021 21:50



Mathematics, 09.10.2021 21:50



