
Chemistry, 24.05.2021 23:30 rivermadds4163
Help plz:)))I’ll mark u Brainliest Part 3 Directions : Identify the type of chemical reaction from the UNbalanced equations : (synthesis single replacement , double replacement, decomposition combustion.)
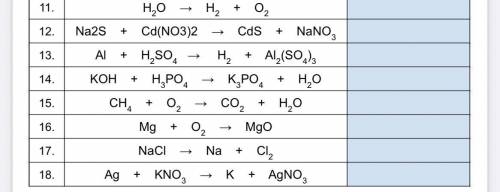

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:50
Why do scientists look for patterns in the world? a. patterns can explain observations. b. patterns never change, no matter what. c. patterns are easy for scientists to detect. d. patterns are all the same, through all time.
Answers: 1

Chemistry, 22.06.2019 12:30
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2
You know the right answer?
Help plz:)))I’ll mark u Brainliest
Part 3 Directions : Identify the type of chemical reaction from...
Questions



Biology, 17.03.2020 06:37



Social Studies, 17.03.2020 06:38



Mathematics, 17.03.2020 06:38


Advanced Placement (AP), 17.03.2020 06:38


Biology, 17.03.2020 06:38

Mathematics, 17.03.2020 06:38

Mathematics, 17.03.2020 06:38







