What volume (in L) of ethane (C2H6) will be
required to produce 42.5 L of carbon dioxide in
t...

Chemistry, 25.05.2021 01:00 FavvBella84
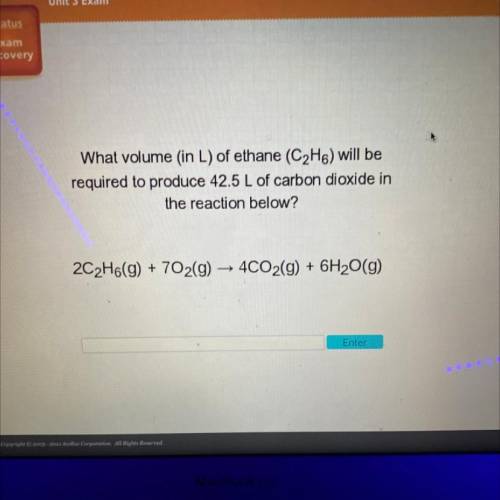
What volume (in L) of ethane (C2H6) will be
required to produce 42.5 L of carbon dioxide in
the reaction below?
2C2H6(g) + 702(g) → 4CO2(g) + 6H2O(g)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 22.06.2019 01:20
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1

Chemistry, 22.06.2019 03:30
Each pair of clay balls represents to planetesimals if each plane test molluscum pound of the same material and is separated by the same distance which pair experiences the greatest gravitational attraction
Answers: 2

Chemistry, 22.06.2019 06:00
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3
You know the right answer?
Questions


Chemistry, 14.12.2020 16:20


English, 14.12.2020 16:20


Mathematics, 14.12.2020 16:20


Mathematics, 14.12.2020 16:20

History, 14.12.2020 16:20

Physics, 14.12.2020 16:20

Engineering, 14.12.2020 16:20



Mathematics, 14.12.2020 16:20

English, 14.12.2020 16:20


English, 14.12.2020 16:20

Mathematics, 14.12.2020 16:20

Health, 14.12.2020 16:20

Biology, 14.12.2020 16:20



