
Chemistry, 25.05.2021 01:00 NetherisIsTheQueen
In an aqueous chloride solution cobalt(II) exists in equilibrium with the complex ion CoCl42-. Co2 (aq) is pink and CoCl42-(aq) is blue. At Low Temperature the pink color pre-dominates. At High Temperature the blue color is strong. If we represent the equilibrium as:
CoCl4^2-(aq) <--> Co2+(aq) + 4Cl-(aq)
We can conclude that:___.
1. This reaction is:___.
A. Exothermic
B. Endothermic
C. Neutral
2. When the temperature is decreased the equilibrium constant, K:.
A. Increases
B. Decreases
C. Remains the same.
3. When the temperature is decreased the equilibrium concentration of Co2:.
A. Increases
B. Decreases
C. Remains the same.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Agood hypothesis includes which of the following? a: prediction b: data c: uncertainty d: conclusion
Answers: 1

Chemistry, 21.06.2019 22:00
The graph above shows how the price of cell phones varies with the demand quantity. the equilibrium price for cell phones is where both supply and demand quantities equal $100, 5,000 5,000, $100
Answers: 2

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 23.06.2019 00:30
What is the chemical formula of magnesium bromide? a. mgbr2 b. mgbr c. mg2br2 d. mg2br
Answers: 3
You know the right answer?
In an aqueous chloride solution cobalt(II) exists in equilibrium with the complex ion CoCl42-. Co2 (...
Questions





Mathematics, 03.12.2020 22:50


Mathematics, 03.12.2020 22:50

Mathematics, 03.12.2020 22:50

Mathematics, 03.12.2020 22:50



Mathematics, 03.12.2020 22:50

Physics, 03.12.2020 22:50

History, 03.12.2020 22:50

History, 03.12.2020 22:50

Mathematics, 03.12.2020 22:50

Mathematics, 03.12.2020 22:50


Engineering, 03.12.2020 22:50

Chemistry, 03.12.2020 22:50

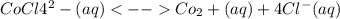
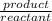 =
=![\frac{[CO^2^+][Cl^-^4]}{CoCl^2^-_4}](/tpl/images/1345/2628/18cbc.png)
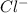 and
and  rises, and K rises as well , thus it increases .
rises, and K rises as well , thus it increases .

