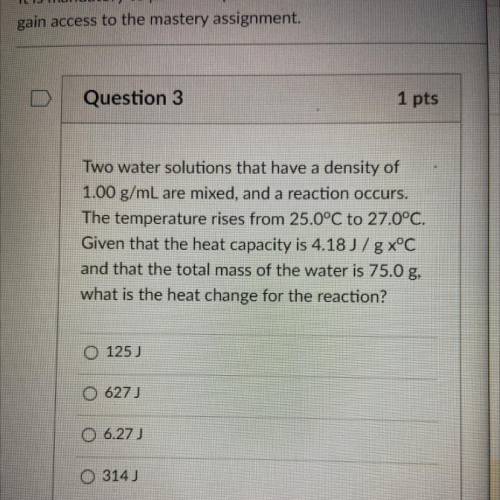
Two water solutions that have a density of
1.00 g/mL are mixed, and a reaction occurs.
The te...

Chemistry, 25.05.2021 08:50 llnapier8924
Two water solutions that have a density of
1.00 g/mL are mixed, and a reaction occurs.
The temperature rises from 25.0°C to 27.0°C.
Given that the heat capacity is 4.18 J/g x°C
and that the total mass of the water is 75.0 g,
what is the heat change for the reaction?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Write two balanced equations 1. dissolving of solid sodium hydroxide in water 2. the reaction of sodium hydroxide solution with hydrochloric acid
Answers: 1

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

You know the right answer?
Questions


Mathematics, 02.01.2020 20:31


History, 02.01.2020 20:31

History, 02.01.2020 20:31

Mathematics, 02.01.2020 20:31

Mathematics, 02.01.2020 20:31




Geography, 02.01.2020 20:31



Social Studies, 02.01.2020 20:31


Biology, 02.01.2020 20:31



Mathematics, 02.01.2020 20:31

Mathematics, 02.01.2020 20:31



