
Chemistry, 25.05.2021 18:40 OkayLearn5522
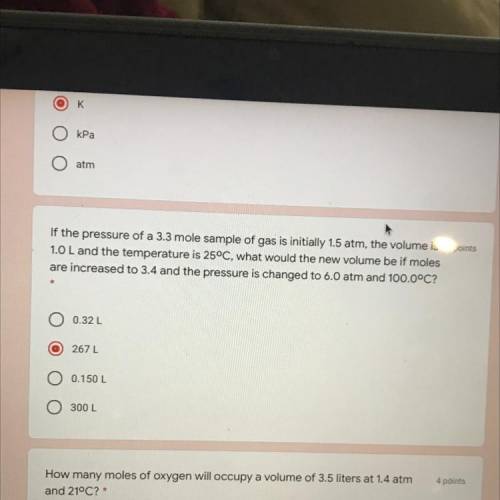
If the pressure of a 3.3 mole sample of gas is initially 1.5 atm, the volume is 4 points
1.0 L and the temperature is 25°C, what would the new volume be if moles
are increased to 3.4 and the pressure is changed to 6.0 atm and 100.0°C?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2
You know the right answer?
If the pressure of a 3.3 mole sample of gas is initially 1.5 atm, the volume is 4 points
1.0 L and...
Questions

Mathematics, 04.03.2021 22:00


Arts, 04.03.2021 22:00

Mathematics, 04.03.2021 22:00

Mathematics, 04.03.2021 22:00

Chemistry, 04.03.2021 22:00


Mathematics, 04.03.2021 22:00


Spanish, 04.03.2021 22:00



Mathematics, 04.03.2021 22:00

Mathematics, 04.03.2021 22:00




Mathematics, 04.03.2021 22:00




