Given the reaction:
4NH3(g) + 502(g) → 4NO(g) + 6H2O(g)
At constant pressure, how many liters...

Chemistry, 25.05.2021 19:20 issacbeecherpebpyl
Given the reaction:
4NH3(g) + 502(g) → 4NO(g) + 6H2O(g)
At constant pressure, how many liters of O2 (g)
would be required to produce 20 liters of NO (g)?
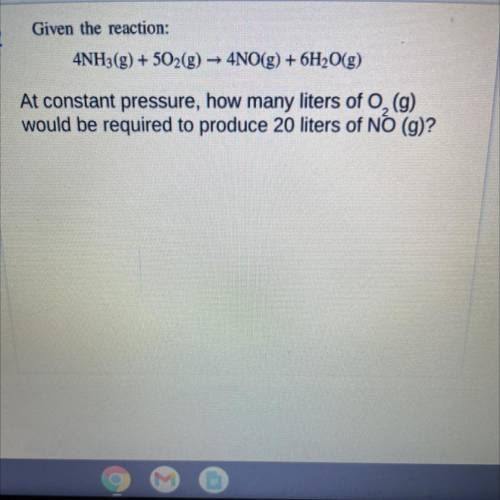

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1


Chemistry, 23.06.2019 06:00
What are the coefficients to balance the following equation? ba+br2=babr2
Answers: 2

Chemistry, 23.06.2019 07:30
Type the letter that represents the correct location for each particle type below. the neutron is found at __ the electron is found at __ the proton is found at __
Answers: 1
You know the right answer?
Questions

English, 03.12.2020 01:00

Mathematics, 03.12.2020 01:00



Mathematics, 03.12.2020 01:00

Mathematics, 03.12.2020 01:00


Biology, 03.12.2020 01:00

Biology, 03.12.2020 01:00

Health, 03.12.2020 01:00


Mathematics, 03.12.2020 01:00

English, 03.12.2020 01:00

History, 03.12.2020 01:00


Mathematics, 03.12.2020 01:00

Mathematics, 03.12.2020 01:00


Chemistry, 03.12.2020 01:00

Mathematics, 03.12.2020 01:00



