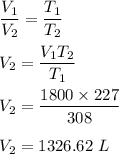Chemistry, 26.05.2021 08:40 zubeenakhtar1386
A good year blimp (a huge airship) is filled to a
volume of 1,800L at a temperature of 35°C. As
the weather changed the blimp is then cooled
at constant pressure to a temperature of -46°C.
What is the final volume of the blimp?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:00
6what is the importance of water on earth? a) it keeps the top layer of the geosphere cool b) it allows life to exist c) it provides ice at the poles d) it creates earth's blue color from space
Answers: 2

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2

Chemistry, 23.06.2019 04:00
What two categories of toxins were present in the air at dish,texas as a result of the gas pipelines that pass through the area
Answers: 1

You know the right answer?
A good year blimp (a huge airship) is filled to a
volume of 1,800L at a temperature of 35°C. As
Questions


Biology, 16.12.2020 23:20


Biology, 16.12.2020 23:20

Arts, 16.12.2020 23:20

History, 16.12.2020 23:20



Mathematics, 16.12.2020 23:20

Mathematics, 16.12.2020 23:20

Arts, 16.12.2020 23:20