
Chemistry, 26.05.2021 09:50 coreycbg1127
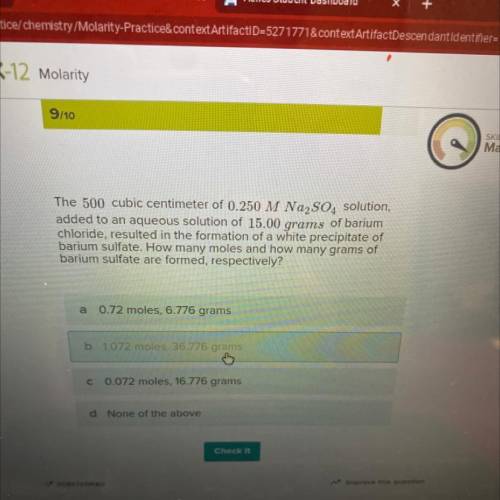
The 500 cubic centimeter of 0.250 M Na2SO4 solution,
added to an aqueous solution of 15.00 grams of barium
chloride, resulted in the formation of a white precipitate of
barium sulfate. How many moles and how many grams of
barium sulfate are formed, respectively?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:00
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2
You know the right answer?
The 500 cubic centimeter of 0.250 M Na2SO4 solution,
added to an aqueous solution of 15.00 grams of...
Questions



Mathematics, 19.01.2020 21:31

Health, 19.01.2020 21:31



Chemistry, 19.01.2020 21:31

Mathematics, 19.01.2020 21:31

Mathematics, 19.01.2020 21:31


Mathematics, 19.01.2020 21:31

Spanish, 19.01.2020 21:31

Social Studies, 19.01.2020 21:31

English, 19.01.2020 21:31



Biology, 19.01.2020 21:31





