
Chemistry, 26.05.2021 18:30 renegade2020
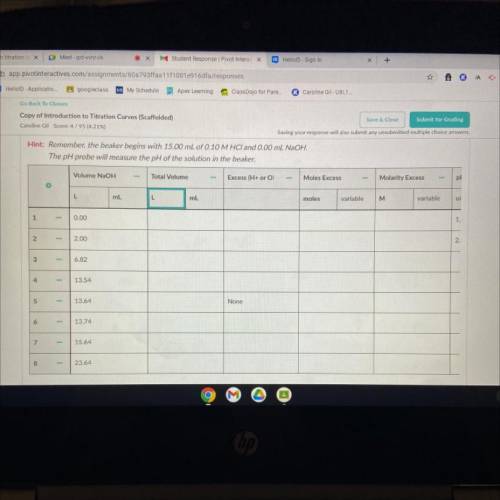
For each volume NaOH, determine the following and record in the data table.
1. Total volume of solution after NaOH is added. (The beaker starts with 15.00 mL HCI)
2. The ion in excess. There is no excess ion at the equivalence point, so leave it blank at that volume.
3. The moles excess reactant (use stoichiometry).
4. The molarity of excess reactant.
5. pH of solution

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 04:00
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1

Chemistry, 22.06.2019 05:20
Why does the sun appear to be the brightest star in the sky? a- its apparent brightness is much greater than other stars. b- it burns more gas, making it brighter than any other star. c- it is the largest star in the galaxy, so it is the brightest star. d- its relative distance to earth is closer than the other stars.
Answers: 1

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1
You know the right answer?
For each volume NaOH, determine the following and record in the data table.
1. Total volume of solu...
Questions



Business, 18.10.2019 02:10





History, 18.10.2019 02:10

English, 18.10.2019 02:10

Physics, 18.10.2019 02:10

Mathematics, 18.10.2019 02:10


Mathematics, 18.10.2019 02:10

Business, 18.10.2019 02:10

Business, 18.10.2019 02:10

Geography, 18.10.2019 02:10



Mathematics, 18.10.2019 02:10

Mathematics, 18.10.2019 02:10



