
Chemistry, 26.05.2021 19:00 kamster911
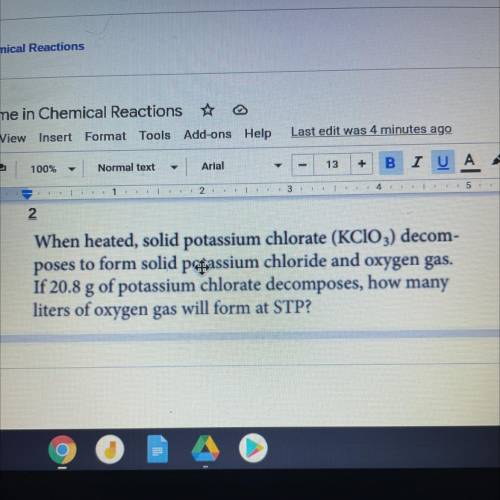
When heated, solid potassium chlorate (KClO3) decom-
poses to form solid passium chloride and oxygen gas.
If 20.8 g of potassium chlorate decomposes, how many
liters of oxygen gas will form at STP?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:50
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 07:00
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3
You know the right answer?
When heated, solid potassium chlorate (KClO3) decom-
poses to form solid passium chloride and oxyge...
Questions

Mathematics, 22.06.2021 06:30




History, 22.06.2021 06:30


Mathematics, 22.06.2021 06:30

Mathematics, 22.06.2021 06:30

Mathematics, 22.06.2021 06:30

Mathematics, 22.06.2021 06:30

Chemistry, 22.06.2021 06:30


Mathematics, 22.06.2021 06:30

Social Studies, 22.06.2021 06:30




Mathematics, 22.06.2021 06:30




